

The postmarketing requirement is a clinical trial that randomizes patients to receive either Andexxa or usual care (the type of care the enrolling institution would provide in the absence of Andexxa). The company advised that continued approval for this indication may be contingent upon postmarketing study results to demonstrate an improvement in hemostasis in patients.
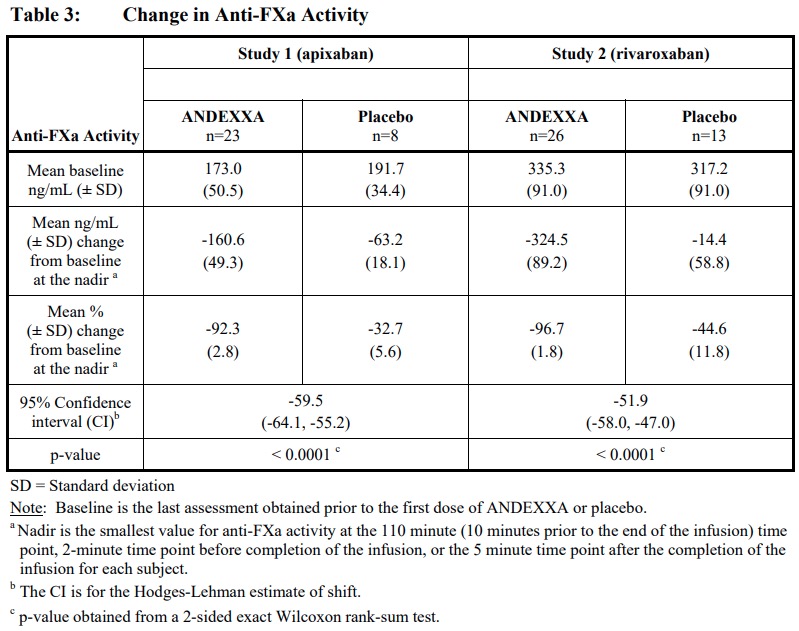
Broader commercial launch is anticipated in early 2019 upon FDA approval of its generation 2 manufacturing process. Portola expects to launch Andexxa under an Early Supply Program with the generation 1 product in early June. recently announced that the US Food and Drug Administration (FDA) has approved the company's Andexxa (coagulation factor Xa, inactivated-zhzo), an antidote indicated for patients treated with rivaroxaban and apixaban when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.Īndexxa received both US Orphan Drug and FDA Breakthrough Therapy designations and was approved under the FDA's Accelerated Approval pathway based on the change from baseline in antifactor Xa activity in healthy volunteers. May 14, 2018-Portola Pharmaceuticals, Inc.


 0 kommentar(er)
0 kommentar(er)
